1. Introduction to Boron carbide ceramics
Boron carbide (B4C) has a hexagonal diamond lattice. There are 12 boron atoms and 3 carbon atoms in the unit cell the chains of atoms are arranged in cubic diagonals. Carbon atoms are active and can be replaced by boron atoms to form a replacement solution and it is possible to break away from the lattice and form defective high boron compounds.

Boron carbide ceramic powder
2. Manufacturing technology of boron carbide ceramics
(1) Preparation of B4C powderThe preparation method of boron carbide powder can refer to the preparation method of SiC powder.
For the preparation of boron anhydride reduced by carbon, this method is to heat the ingredients indirectly or to pass the current directly through the ingredients in the resistance furnace and arc furnace to a temperature of 2200℃
B4C breaks down into carbon-rich and boron, which evaporates at high temperatures. B4C produced in arc furnace contains a large amount of dissociation graphite, the content of 20%~30%. B4C, prepared in a resistance furnace, contains a small amount of free carbon and a small amount of free boron, Up to 1%~2%.
(2) Molding and sintering of boron carbide ceramics
Boron carbide ceramics can be formed by various methods. Check manufacturing methods of silicon carbide ceramics for details. In order to get dense B4C, generally should be made by hot pressing sintering method. The B4C can reach 98% of the theoretical density, which is true when prepared air or ordinary hot pressing furnace, hot pressing temperature is 2100℃, pressure is 80~100MPa, heat preservation for a few minutes, cooling need to maintain the pressure. Due to the poor thermal shock resistance of B4C, the temperature drop should be slow. Hot pressing temperature should not be too high, to 2150℃ will appear B4C-C eutectic liquid phase, but the temperature is too low, the product density is low. Boron carbide ceramics with high density and hardness can be obtained by using B4C superfine material.
3. Properties and applications of boron carbide ceramics
The remarkable characteristic of boron carbide ceramics is its high hardness, only to diamond and cubic crystal BN. Its grinding efficiency can be achieved.
60%~70% of diamond is greater than 50% of SiC, which is 1~2 times of corundum grinding capacity. It has good acid and alkaline resistance and thermal expansion small expansion coefficient (4.5x10 ~6/℃), can absorb thermal neutrons, but poor impact resistance. The use of boron carbide ceramics its hardness is large It can be used as abrasive, cutting tools, wear-resistant parts, nozzles, bearings, axles, etc. With its good thermal conductivity, low coefficient of expansion and ability to absorb thermal neutrons, high temperature heat exchangers and control agents for nuclear reactors can be manufactured. It can be used to make chemical utensils, melting metal crucible and so on.
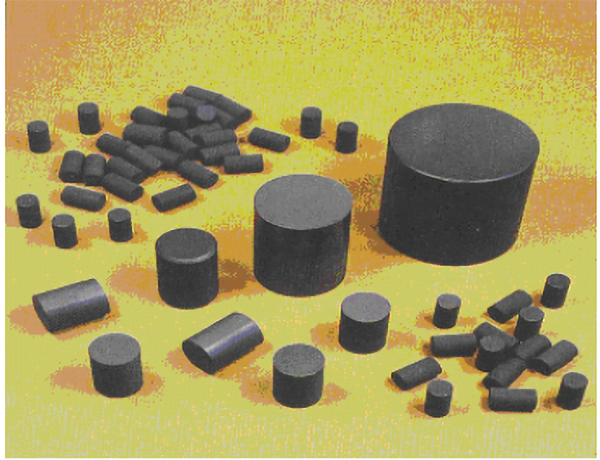
Boron carbide neutron absorber
Declaration: This article is provided by CERADIR™ users or obtained from Internet, the content does not represent the position of CERADIR™. We are not responsible for the authenticity/accuracy of the article, especially the effects of the products concerned. This article is for study only, it does not constitute any investment or application advice. For reprinting, please contact the original author. If it involves the copyright and/or other issues, please contact us and we will deal with it asap! CERADIR™ has the interpretation of this declaration.







